Abstract
Adoptive T cell therapy has been successful in boosting viral-specific immunity post-HSCT, preventing viral rebound of CMV and EBV. However, the therapeutic use of T cells to boost HIV-specific T cell immunity in HIV+ patients has been met with limited success. Despite multiple attempts to eradicate HIV with allogeneic HSCT, there is only one case of functional HIV cure post-HSCT, the Berlin Patient. Hence, we hypothesized that broadly HIV-specific CD8 and CD4 T-cells (HXTCs) could be expanded from patients on ARVs, as well as HIV negative adult (dHXTCs) and cord blood donors (cbHXTCs), employing a non-HLA restricted approach for the treatment of HIV+ individuals after autologous or allogeneic HSCT.
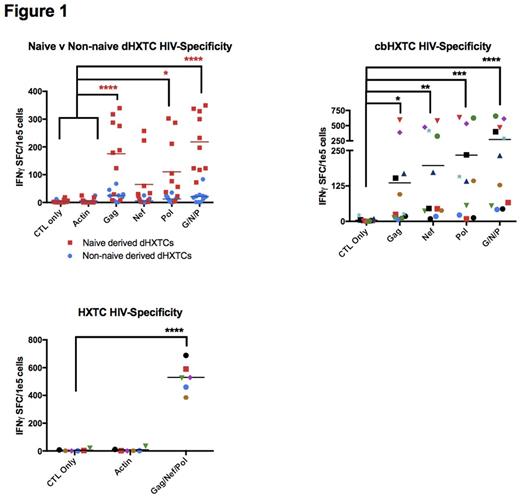
We have expanded autologous HXTCs from HIV+ subjects under NCT02208167. To extend this approach to the allogeneic HSCT setting, we generated dHXTCs from HIV naive adults (n=8) and cbHXTCs from cord blood donors (n=8). IFN-gamma ELISPOT showed dHXTCs were specific against Gag (mean=331.25 SFC/105cells) and Nef (mean=242.63) versus irrelevant antigen (mean=13 SFC) (n=8). Similarly, we are able to produce cbHXTCs (n=8) that showed specificity to Gag (mean=103.252 SFC/105cells), Nef (mean=109.43), or Pol (mean=130.56), (p<0.0001 compared to controls) in IFN-gamma ELISPOT. dHXTC products displayed polyfunctionality, producing proinflammatory TNFα, IL2, IL6, IL8, and perforin responses (p<0.05) to HIV stimulation. Importantly, dHXTCs (p=0.0004) and cbHXTCs (p=0.0003) were able to suppress HIV replication compared with nonspecific CD8+ T cells when co-cultured with autologous CD4+ T cells infected with HIV SF162 at an Effector-to-Target ratio of 20 to 1. Exhaustion marker analysis of cbHXTCs products revealed minimal expression of PD1, TIM3, LAG3, KLRG1, and CD57 (n=3). Epitope mapping revealed that dHXTC and cbHXTC products contained T cells recognizing unique epitopes not typically identified in HIV positive individuals, which may be critical in overcoming viral immune escape post-HSCT.
In summary, HIV-specific T cells can be expanded from HIV positive and HIV negative donors for clinical use. Focusing on donors with HLA types that are associated with well characterized HIV responses (HLA A02) or associated with delayed progression to AIDS (HLA B27, B51, B57) may allow us to identify HLA restricted epitopes critical for the successful development of a potent HIV-specific T cell therapeutic. Hence, the administration of HXTCs derived from virus naive donors could offer a unique curative strategy post-allogeneic HSCT and cord blood transplant.
Bollard: Torque: Membership on an entity's Board of Directors or advisory committees; Cellectis: Membership on an entity's Board of Directors or advisory committees; Neximmune: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal